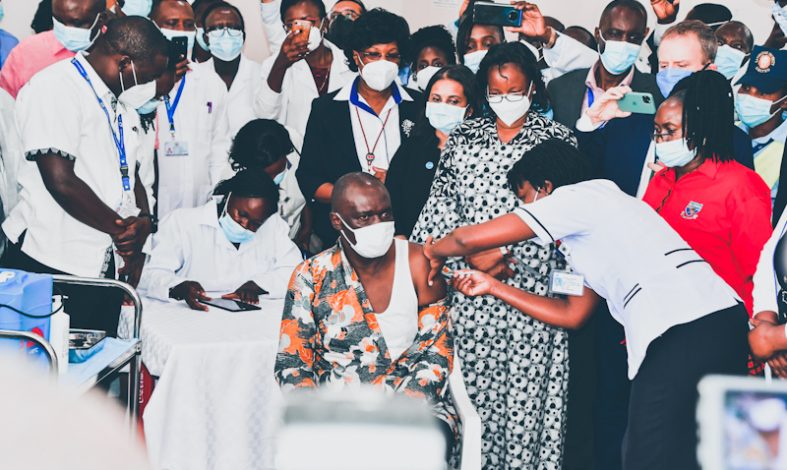
Kenya has launched the most advanced human trial for Rift Valley fever (RVF) in an outbreak-prone region, a milestone in global epidemic preparedness.
On July 8, 2025, researchers at the KEMRI–Wellcome Trust Research Programme in Kilifi, in partnership with the University of Oxford, began Phase II clinical trials of the ChAdOx1 RVF vaccine. The study is funded by the Coalition for Epidemic Preparedness Innovations (CEPI).
Why This Trial Matters
- First-ever Phase II RVF vaccine trial in an endemic country
- 240 adult volunteers (ages 18–50) enrolled from Kilifi’s Health and Demographic Surveillance System area
- Builds on successful Phase I trials in the UK and Uganda, which showed strong immune responses and safety
The ChAdOx1 RVF vaccine uses the same viral vector technology as the Oxford–AstraZeneca COVID-19 vaccine, credited with saving millions of lives globally.
Vaccine Administration and Goals
The study is enrolling 240 adult volunteers aged 18 to 50 from the Kilifi Health and Demographic Surveillance System area, a region that has experienced severe RVF outbreaks in the past. Participants will receive either one or two doses of the vaccine or a control vaccine. The trial aims to assess the vaccine’s safety, immune response, and durability in an endemic setting.
Earlier Phase I trials conducted in the UK and Uganda showed the vaccine to be safe and highly immunogenic, with volunteers developing strong antibody and cellular immune responses. If successful, the ChAdOx1 RVF vaccine could become the first licensed human vaccine against Rift Valley fever.
Rift Valley Fever: A Climate-Sensitive Threat
RVF is a mosquito-borne zoonotic disease that spreads through:
- Contact with infected animals (e.g., sheep, goats, cattle)
- Bites from infected mosquitoes
While most cases are mild, severe forms can cause:
- Blindness
- Convulsions
- Encephalitis
- Haemorrhagic symptoms with up to 50% mortality
First identified in Kenya’s Rift Valley, RVF has since spread across Africa and into the Middle East. Experts warn that climate change, through altered rainfall and expanding mosquito habitats, could increase the frequency and severity of outbreaks.
One Health Potential: Protecting People and Livestock
Preclinical studies show the vaccine also protects livestock, including sheep and goats. This opens the door to a One Health approach, safeguarding both human and animal health.
“Nearly 100 years after Rift Valley fever was discovered, there are still no approved vaccines or treatments. This trial brings us closer to addressing the rising frequency of outbreaks,” Prof. George Warimwe, Principal Investigator, KEMRI–Wellcome Trust
“Investing in this promising vaccine gives us a greater chance at protecting vulnerable populations against a threat that may become more prevalent with climate change,” Dr. Richard Hatchett, CEO, CEPI.
“The launch of a Phase II trial in an endemic country is a crucial milestone. The ChAdOx1 RVF vaccine offers hope to communities affected by climate-driven disease,” Dr. Jean Kaseya, Director General, Africa CDC.
Kenya’s Role in Global Health Innovation
This trial reinforces Kilifi’s reputation as a hub for world-class medical research and highlights Kenya’s leadership in vaccine development and epidemic response.
If successful, the ChAdOx1 RVF vaccine could become the first licensed human vaccine against Rift Valley fever, offering dual protection for health and livelihoods across East Africa.